6/1/2022
pH Correction for Irrigation Water
José Rodriguez
Most growers know that correcting pH is important, but they don’t always know why. When we correct the pH in irrigation water, we’re actually adjusting the irrigation water alkalinity, but why are we doing that?
To understand, we need to define pH. In simple terms, the pH of a solution relates to the concentration of hydrogen cations (H+). The pH scale goes from 1 to 14, where the lower the pH (high H+ concentration), the more acidic; the higher the pH (low concentration of H+), the more basic.
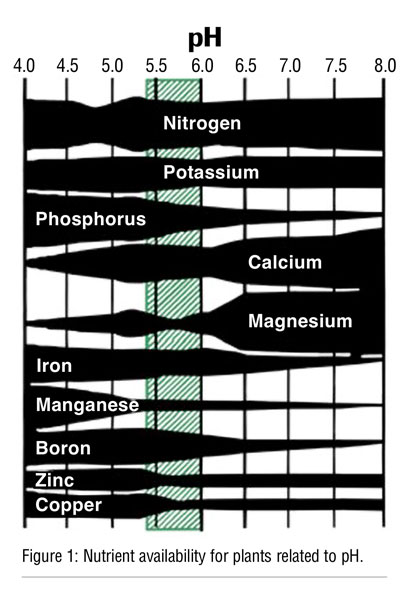
Why is irrigation water pH important in CEA? The nutrient availability for plants is affected by pH (Figure 1). Whereas iron (Fe), manganese (Mn), boron (B), zinc (Zn) and copper (Cu) are more available at low pH values, calcium (Ca) and magnesium (Mg) are more available at higher pH values. At very low pH, however, the increase in Fe, Mn and Al (aluminum) can become toxic. Therefore, between 5.5 and 6.2, most micronutrients are available at proper levels.
Why do we correct irrigation water alkalinity? The concept “alkalinity,” is a measure of the resistance of a water solution to change its pH. When acid is added to the irrigation water, the H+ of the acid first react with the carbonates and bicarbonates on the water forming CO2 and water. Initially, pH decreases slowly. When no more alkalinity is left, the pH will drop abruptly.
We measure alkalinity in ppm. Even though it’s closely related to pH, two different water samples could have similar pH values, but different levels of alkalinity. It’s going to take much more acid to lower the water sample’s pH with more alkalinity than the one with less.
Carbonates and bicarbonates brought by the irrigation water will react with H+ in the growing media and increase its pH, lowering the micronutrient availability. Plants will suffer micronutrient deficiencies, but completely removing the alkalinity isn’t necessarily good. Most fertilizers are acidifying in nature; if our irrigation water doesn’t have any alkalinity, the acidifying fertilizer can lower the growing media pH to toxic levels. This case can be observed with Reverse
 Osmosis (RO) water, where all alkalinity was removed and potassium bicarbonate is needed to increase the alkalinity to a desirable value of 60 to 70 ppm.
Osmosis (RO) water, where all alkalinity was removed and potassium bicarbonate is needed to increase the alkalinity to a desirable value of 60 to 70 ppm.
We correct pH (mostly lower it) to correct alkalinity and optimize micronutrient availability. In some cases, we start with water that has no alkalinity. Then we need to adjust the pH up due to the natural acidifying quality of most fertilizer formulas.
Our injectors correct pH by proportionally injecting acids or bases for your fertilizer formula blend. How much? And how? Ask a professional to get the right balance for you. IG
José Rodriguez is an agronomist with a Ph.D. in Engineering Sciences and is Business Development Manager for Dosatron/Dilution Solutions. He can be reached at jose.rodriguez@dosatronusa.com and (863) 258-6888.